Reckitt/Mead Johnson Nutrition Baby Formula Recall
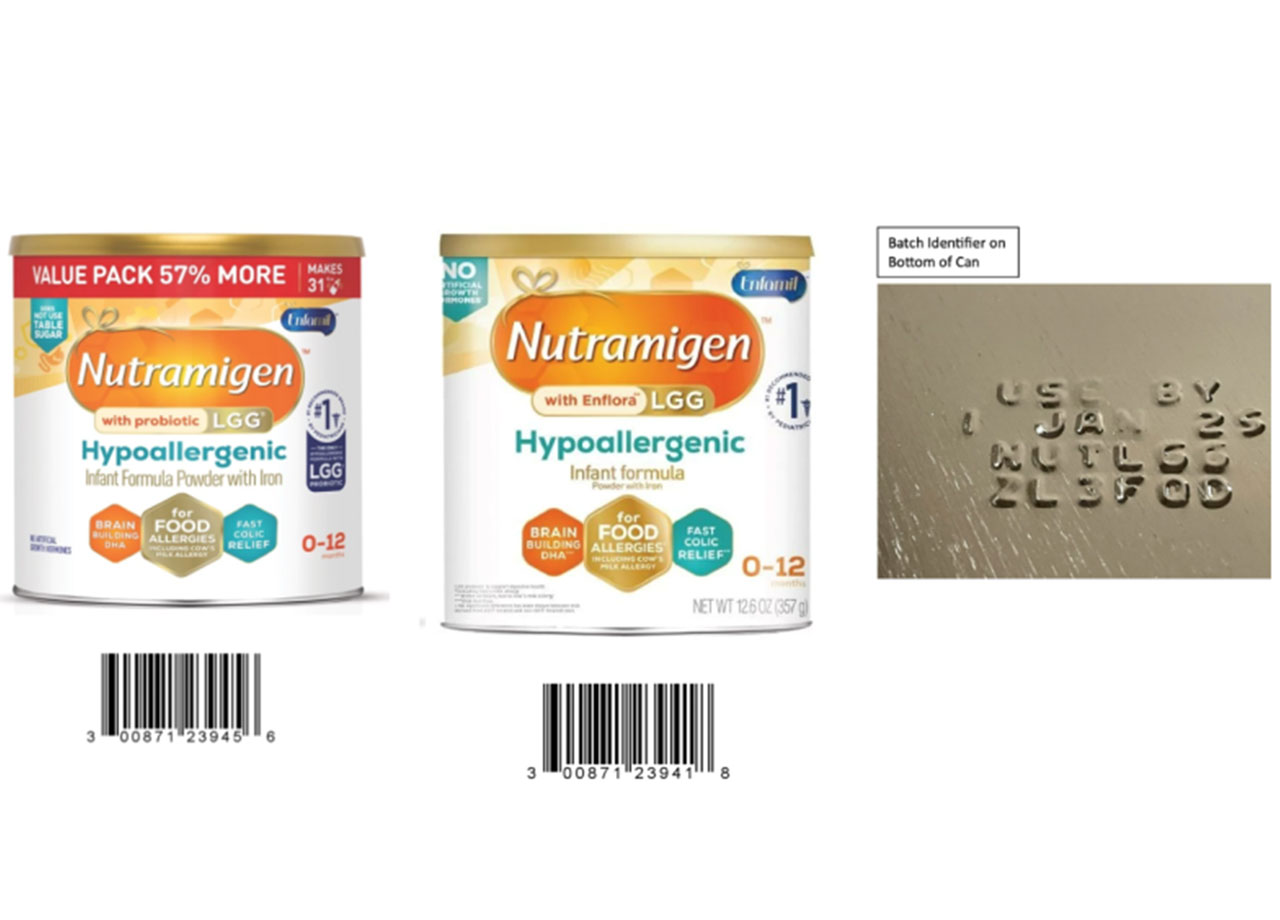
According to the FDA, the following Reckitt/Mead Johnson Nutrition Nutramigen baby formulas have been recalled "out of an abundance of caution" after Israeli health authorities confirmed a type of bacteria called Cronobacter sakazakii was found in cans that were being important to Israel from the U.S. The recall includes 675,030 cans sold in the U.S. that were produced in Zeeland, Michigan starting in June 2023.
While the cans for the U.S. market had been manufactured separately, and all tests conducted by the FDA have come back negative, Reckitt/Mead Johnson is issuing the recall anyway. Here are the batch codes of the affected products:
ZL3FHG (12.6 oz cans)ZL3FMH (12.6 oz cans)ZL3FPE (12.6 oz cans)ZL3FQD (12.6 oz cans)ZL3FRW (19.8 oz cans)ZL3FXJ (12.6 oz cans)
However, Reckitt/Mead Johnson reports that "Based on the limited availability of the remaining stock of this special infant formula, it is believed that much, if not all, of the products recalled in the United States have been consumed." Symptoms can include poor feeding, irritability, temperature changes, jaundice, grunting breaths, and abnormal movements. If your baby is exhibiting any of these symptoms, call your pediatrician immediately.
Customers can contact the company for a refund by calling 866-534-9986 or by emailing consumer.relations@rb.com.


